Harnessing Memory’s Malleability to ‘Rewrite’ Fear and Other Negative Memories
Dr. Ramirez and colleagues have identified the location of individual memories in the rodent brain, both positive and negative, and have artificially activated such individual memories. Remarkably, they have also succeeded in modifying negative memories in ways that they hope can inform the development of new therapies in people for memory based illnesses such as post-traumatic stress disorder.
Steve Ramirez, Ph.D.
Assistant Professor of Psychological and Brain Sciences
Boston University
2016 BBRF Young Investigator
Even before Dr. Steve Ramirez begins to tell you about the experiments his team has conducted in the last few years—remarkable experiments that he acknowledges sound to some like the stuff of science fiction—he has a winning way of drawing you into the subject he’s spent his career studying.
“I gently poke fun at my physicist friends,” he says, “by reminding them that each of us, between our ears, has the one thing that they tell us we cannot have. We each possess a time machine that can instantly transport us to the past. All you have to do is close your eyes and think about something as simple as what you had for breakfast today, or as complex as how you felt when you visited your grandmother’s house when you were a child. That’s it! In the blink of an eye, you’ve time-traveled back to a moment in your past, without breaking a sweat. It’s impressive the brain can do that!”
A 2016 BBRF Young Investigator, Dr. Ramirez, who earned his neuroscience doctorate at MIT, is a faculty member at Boston University and a member of BU’s Center for Systems Neuroscience. It is the goal of his lab to reveal the neural-circuit mechanisms of memory storage and retrieval, and to artificially modulate memories in ways that might relieve the those who have experienced severe trauma, or to enhance the recall ability of people with memory impairment.
Over the last decade, Dr. Ramirez and colleagues have published a series of research papers in leading scientific journals including Neuron, Nature, and Science describing their successful attempts to identify the location of individual memories in the rodent brain, to artificially activate such individual memories, and perhaps most remarkably, to modify them in ways they hope can inform the development of new therapies for memory-based illnesses in people.
“When you’re absorbed by a memory, when it’s good, it can feel great, and when it’s bad it can be pretty debilitating,” Dr. Ramirez notes. “So memory has this kind of bi-directional power to put us in an unbelievably positive head-space or put us in the darker corners of what past experiences have left us with in terms of the marks they make in our brain.”
WHERE DO MEMORIES LIVE?
Before he could seriously contemplate the prospect of therapeutically modifying memories, Dr. Ramirez’s first goal was to work on a problem that many others in the field have worked on, including his mentor at MIT, Dr. Susumu Tonegawa. Where exactly are memories located in the brain? Can we figure out a way to visualize a memory as it is being formed?
Dr. Ramirez’s general approach to the problem, he explains, was a bit like trying to figure out from across the street who is working at night in an office building. Your attention is directed to the offices in which the lights are on, where there appears to be activity. Reduced to its fundamentals, this is what he and his colleagues did with mice. At moments in time when it was reasonable to assume a mouse was forming new memories, the team looked into the brain to see which neurons were being activated or whose level of activity was elevated relative to other neurons in their neighborhood.
They made these observations at specific moments—when, for example, a mouse was put in contact with a member of the opposite sex—typically, the basis for a positive memory. Or, they put the animal in a special cage in which tiny shocks that feel like static electricity are randomly experienced. These are not painful but they are uncomfortable. When animals feel these little shocks, they freeze in place—a mark that they have been taken by surprise and have paused to contemplate how to protect themselves. This gets recorded as a negative memory.
The task of searching for specific neurons activated at such moments is very challenging, for many reasons. First, there are an estimated 70 million neurons in the mouse brain; large numbers of them are constantly being activated, for an enormous number of possible reasons. Many operations are going on at the same time. On the one hand, forming a new memory surely only occupies a small subset of brain cells. On the other hand, it is impossible to know, by simply observing them visually, which activated neurons at any given moment are being recruited for encoding a memory.
The task is somewhat more manageable if you concentrate on a brain structure already known from past research to be central in memory formation, retention, and recall. The team focused on the hippocampus. The mouse brain, like that of humans, contains two, one on each side of the brain. They are crescent-shaped structures that are centrally involved in encoding memory. It’s long been known that if one disables or removes the hippocampus of an animal, or if the hippocampus is damaged, say, due to a brain injury, memory is severely impaired.
But the hippocampus is itself a complex structure, packed with neurons that are arrayed in multiple layers. What the Ramirez team did was figure out a way to use genetic footprints to identify which neurons have just been activated. Activation alters gene expression in particular ways, and researchers can see these in real-time by linking the gene changes with chemical tags that glow in fluorescent colors. It’s what Dr. Ramirez calls “a trick we use to get a read-out of which brain cells are active, specifically when animals are making or forming memories.”
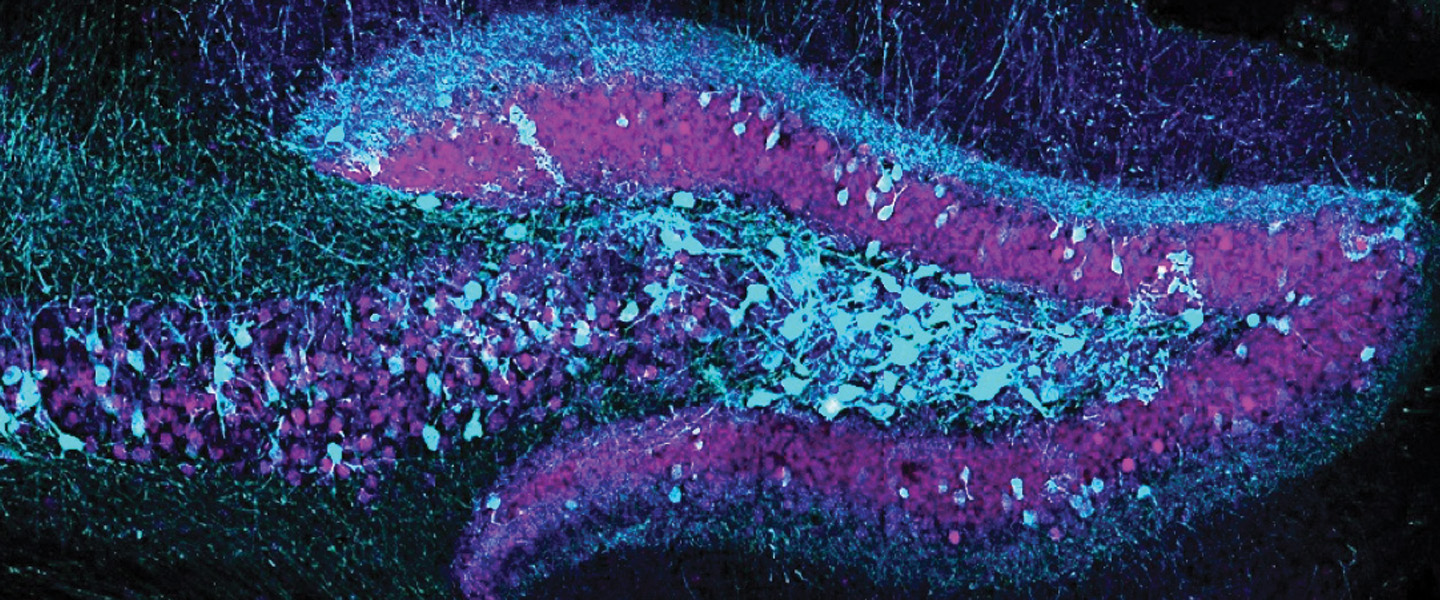
Using fluorescent tags of neural activation at moments during which memories were being formed enabled the team to literally show what Dr. Ramirez calls “the crystallization of a memory.” He describes the picture shown on page 5: “This is one of my favorite images that I’ve ever taken as a scientist,” he says. “These are cross-sections of the hippocampus. The cells that show up as green dots in the top image are hippocampal cells that we believe are holding on to a negative memory.” These cells became illuminated while a caged rodent was experiencing mild foot shocks. Below, the cells that display a blue color “are the ones that we think are holding on to a positive memory”—for example, a pleasurable social encounter or food treat.
After he obtained these images, Dr. Ramirez paused to reflect. “Memory, as we experience it, is this ephemeral thing, this subjective thing— something that doesn’t seem like something you can reach out and touch.” And yet the images from the hippocampus speak to another thing we intuitively believe to be true: that memories must on some level be a physical phenomenon, that they must have some physical basis in the brain.
The physical manifestation of memory—the constellation of neurons that happens to be activated while a memory is being recorded and stored —is called an “engram” by scientists. No one knows how many neurons are recruited to form these constellations. And it is almost certain that individual neurons can be part of different engrams—they are part of multiple memories. Another important fact about the physical manifestation of memories: according to Dr. Ramirez, they are “distributed” in the brain. The engrams representing positive and negative memories depicted on this page are located in the rodent hippocampus, but there are other dimensions of these and all memories that engage neurons in locations scattered across the brain. For example, we know that memories trigger our emotions; the portion of an engram that contains the memory’s emotional “coloring” involves a brain structure called the amygdala, which is also activated when the memory is encoded or subsequently recalled.
ARTIFICIALLY ACTIVATING A MEMORY
Once Dr. Ramirez and his team succeeded in visualizing specific memories in the mouse brain, the next step was to see whether they could artificially activate them. That is, when they wanted to, as opposed to when a rodent might just happen to recall the memory. To do this, they used a technology called optogenetics co-developed at Stanford in the early 2000s by BBRF Scientific Council member, grantee, and prize-winner Dr. Karl Deisseroth, and colleagues. Optogenetics involves making specific neurons in the rodent brain sensitive to a particular wavelength of light. Threadlike fiberoptic wires are introduced into the animal’s brain to deliver the beams of light to the desired neurons. When the light is delivered, the neurons are activated.
The team performed a fascinating experiment with a mouse that had been allowed to explore a cage in which it experienced mild foot shocks, causing it to freeze in fear and thus forming a negative memory of that place. They identified the engram of that memory in the animal’s hippocampus. The next day, the same animal was placed in a cage with “a completely different environment, with completely different sights, sounds and smells associated with it.” The animal had no reason to be fearful of this second cage—nothing unpleasant occurred there.
Then what Dr. Ramirez calls “the million-dollar experiment”: While the animal was in the “safe” cage, they used optogenetics to activate the cells holding the negative memory formed the day before in the first cage. Result: the animal “almost immediately goes into a freezing posture” even though it was under no threat at all.
This was a proof-of-principle for the team “that really opened up the floodgates.” They now knew they could conduct experiments in rodents in which they could attempt to manipulate memories. One set of experiments could test, for example, whether artificially activating the “fear” memory when the animal was in a variety of different environments always generated the same freezing behavior. Or might that behavior change in some environments?
In fact, when they placed a rodent like the one described above in a very large, open box, much larger and less confining than a cage, it did not freeze at all, but rather seemed to look for ways to escape. It’s analogous, says Dr. Ramirez, to a person who sees a grizzly bear at close quarters in the woods compared with on the far side of a broad, fastflowing river. The context does affect one’s response.
This brings to mind another important fact about memory that previous research has firmly established. We know that memories are constantly being modified and updated with new information. Put another way: we know from experience that memory is malleable. This can be a good thing or a bad thing, depending on the circumstances. It’s good in the sense that all memory involves an act of learning. The brain is taking in information about an event from the senses, remembering all sorts of things about the context, and laying down memories for subsequent retrieval. This is how memory can be considered among the most important “adaptive” capabilities of the brain, in the evolutionary sense; we remember what we have enjoyed and benefited from, and we remember what has given us pain and put us in harm’s way.
At the same time, memory can make us virtual prisoners of our past. For example, the soldier who has experienced trauma or the child who has been physically abused. In PTSD, memories of the trauma intrude upon consciousness, and can paralyze the individual.
REWRITING MEMORIES
The question that really intrigued Dr. Ramirez was: “Can we leverage memory’s malleability? Is it possible that we could utilize the malleability of memory in a therapeutic way?” An important existing therapy for PTSD does try to do that. In exposure therapy, the doctor, under controlled conditions, works with the traumatized patient to expose them to a stimulus that causes fear, but in a safe environment. When this works, the patient gradually learns to avoid being triggered by the stimulus.
Dr. Ramirez, working with rodents, wanted to explore a very different approach. He knew that he could identify specific memory traces in the brain and that he could activate them artificially. The next question was: could the team actually modify an already-formed memory in the rodent brain? They set out to create what they called a “false memory.”
This involved taking advantage of the process through which a memory is updated. Researchers call this process “reconsolidation.” Every time we recall a memory, it can and often is altered in some way to reflect new information or the context in which it has just been recalled. It is thought, incidentally, that this naturally occurring process of memory modification is what can make some memories unreliable or unfaithful to the original context in which they were formed.
The intervention designed by Dr. Ramirez’s team involved taking an animal that had formed a neutral memory of a safe environment and then activating the engram associated with that memory precisely when the same animal was subjected to some unpleasant foot shocks. “We wanted to know if their memory of the safe environment and the experience of getting those shocks could become linked or connected at the biological level,” Dr. Ramirez explains.
“The pretty remarkable thing was that when we put the animal back in a safe environment, now it seemed to be afraid of it. Absolutely nothing bad had happened to it there, but we had effectively updated the memory of that safe place with the memory of getting the foot shock.” In a sense, the animal now had a “false memory” of the safe place, associating it now with danger.
There were other experiments. In one paper, the team described activating a positive memory in animals that had been conditioned to experience chronic stress. Activation of the positive memory tended to diminish the behaviors in the animals that are associated with depressive behavior. “This is a case where we apply what we know from humans to what we see in the animal experiments,” Dr. Ramirez says. “There are a whole host of human studies suggesting that recalling positive memories can buffer the effects of stress. It can reduce levels of stress-associated markers in the blood or get heart rate back to baseline, or even activate the brain’s reward circuitry so that things feel good.”
Drugs can be given to stimulate the reward system to alleviate the negative effects of stress. Dr. Ramirez hopes to find a way to use memory, in this sense, as a drug, perhaps in lieu of administering drugs. A glimpse of this possibility is offered in a paper the team published in Nature Communications in September 2022.
They tagged neurons in a part of the mouse hippocampus called the dentate gyrus with fluorescent markers; these neurons were of three kinds: associated with positive, neutral and negative experiences. The animals underwent fear conditioning. Then, neurons encoding memories in each of the three groups were artificially activated via optogenetics, while fear memories were being recalled.
The team found that during the “window of reconsolidation” when the fear memory was being recalled, if the team artificially activated a competing positive memory, the animals’ conditioned fear behavior virtually disappeared. In effect, the fear memory was overwritten, or rewritten in such a way that it was no longer a memory that triggered fearful behavior. Just as impressive, this modification appeared to be longlasting, perhaps even permanent. The same animals were tested weeks later (a long time in the life of an animal that lives only 2 or 3 years) and the animals remained unafraid of what they previously had feared.
TRANSLATING TO PEOPLE
Since optogenetics can not be used in people, do these experiments have any application in the development of new therapies? Dr. Ramirez thinks they do.
The point he stresses is that we (obviously) cannot make suggestions to rodents when we want to trigger a specific memory they have formed. “But in people I don’t have to go in and optogenetically tinker with anything to get a memory to come online. I could just ask you.”
“We could either try to mimic the effects we saw in mice by giving certain drugs, or ask people to recall certain experiences in an attempt to try to get the brain to fix itself, so to speak. That is really the most exciting part of this work, for me. We showed we could artificially activate positive memories to alleviate aversive states in animals, but to do that in humans, in principle, is as straightforward as asking someone to mentally re-live one of their most cherished positive memories.”
Especially important, it seems, is the malleability of memory in the period of reconsolidation, once the memory has been recalled. In people, this window opens within minutes of recall, and remains open for as long as 6 hours, Dr. Ramirez says. “I think of it as the brain’s window of opportunity for doing therapeutic things with a memory. I think it has tremendous untapped potential. We are learning through this research to take advantage of what we may have thought was one of the ‘bugs’ of memory—that it’s changeable, sometimes not reliable—and we’ve spun it into a ‘feature,’ something that we can leverage therapeutically for the good.”
In their recent paper on rewriting a fear memory in rodents, Dr. Ramirez and colleagues made a potentially very important observation. They found that they could therapeutically modify a fear memory not only by activating a specific memory engram, but also when they used optogenetics to randomly stimulate neurons in a patch of the dentate gyrus of the hippocampus.
One of the possible implications is that it may be possible to stimulate, for example, positive memories while negative memories are being recalled via currently exiting methods of brain stimulation, provided they were focused in the right place and can reach that far into the brain. It might be tested in animals using deep-brain stimulation, Dr. Ramirez says. But that is an invasive procedure and cannot be a typical therapeutic approach for people with PTSD, for example. It is possible, however, that non-invasive brain stimulation methods like TMS (transcranial magnetic stimulation)— now being given to many thousands of patients worldwide to treat depression and several other psychiatric disorders—could be used to stimulate carefully targeted areas that might activate positive memories during fear-memory reconsolidation.
Referring to the success they had in randomly stimulating dentate gyrus neurons to rewrite a fear memory, the team noted: “We believe this manipulation…may work similarly to other stimulation-based protocols associated with neuroplasticity, such as electroconvulsive therapy, deepbrain stimulation, and transcranial magnetic stimulation. We think activating a large set of randomly labeled dentate gyrus cells may perturb the system in a way that provides a ‘reset signal.’”
The team will continue to study in rodents the cellular mechanisms that are engaged when memories are reconsolidated and modifiable. Dr. Ramirez explains: “Is there activity in other brain areas that is changing? Changing in what specific ways? Are there certain receptors in brain cells that are being recruited or blocked, and do we have drugs available that can modify those receptors? That is the kind of conceptual scaffold that this work sets up. The more futuristic approaches we think about would be guided interventions in the brain, whether via stimulation or drugs, but with the aim of jumpstarting processes that can produce therapeutic effects in illnesses affecting memory.”
Written By Peter Tarr, Ph.D.
Click here to read the Brain & Behavior Magazine's April 2023 issue