Accelerating Psychiatric Drug Development
Andrew D. Krystal, M.D.
Professor of Psychiatry
University of California, San Francisco
1997, 1993 BBRF Young Investigator Grant
I have come to appreciate that there are some fundamental flaws in the way that we have tried to develop drugs.” The speaker is Andrew D. Krystal, M.D., a 1997 and 1993 BBRF Young Investigator who is a professor of psychiatry at the University of California, San Francisco.
Dr. Krystal and a team that included 12 others who have received BBRF grants and prizes or serve on BBRF’s Scientific Council, made news in the past year when they successfully demonstrated an alternative approach to drug development that stresses weeding out, at the earliest possible stage, candidate compounds that are less likely to succeed.
Ironically, in taking this unconventional approach focusing on the early elimination of weaker candidates, Dr. Krystal and colleagues generated impressive positive evidence for a first-ever drug to treat anhedonia, a major symptom of several common psychiatric illnesses including depression, anxiety, and PTSD. People with anhedonia are unable to experience pleasure. Across disorders, those with anhedonia tend to have poorer outcomes and a higher risk of suicide.
The unusual approach taken by Dr. Krystal and team was the first comprehensive test of a concept launched years ago by the National Institute of Mental Health (NIMH), dubbed “Fast-Fail.” In the words of NIMH Director Joshua A. Gordon, M.D., Ph.D., a member of the BBRF Scientific Council and 2003 and 2001 BBRF Young Investigator, “The Fast-Fail approach aims to help researchers determine—quickly and efficiently—whether targeting a specific neurobiological mechanism has the hypothesized effect and is a potential candidate for future clinical trials.”
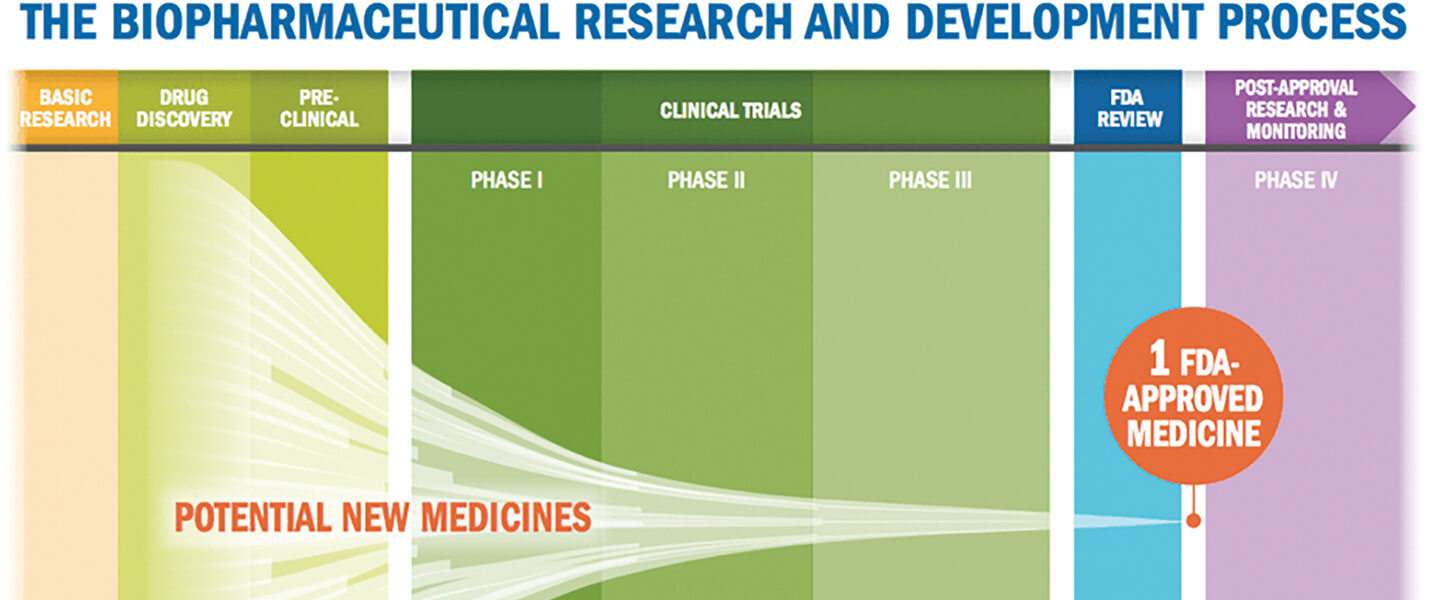
DAUNTING NUMBERS
The need for new approaches to drug development is dramatized by the statistics. According to the FDA, 70% of the drug candidates that manage to survive years of “preclinical” testing in test tubes and animals to enter Phase 1 human “safety” trials make it into Phase 2. In Phase 2, the drug is given to a comparatively small number of individuals affected by the condition the drug is supposed to address. But only 30% make it through Phase 2 and are advanced into Phase 3 trials which involve much larger patient populations—in other words, 21% of the drugs that entered Phase 1. Then, in Phase 3, only a quarter to a third of tested compounds meet the standard of effectiveness, which is typically pivotal for FDA approval. In the end, then, only 5% to 7% of drugs that entered Phase 1 meet their endpoints for effectiveness in Phase 3.
This great funneling process is even starker in its winnowing effect with drugs that are designed to affect the body’s central nervous system (CNS), which includes the brain. Psychiatric drugs are among those in this subset. According to a 2019 report published in Nature Reviews Drug Discovery, based on data from 2010–2017, only 3% of compounds that enter Phase 1 testing in the CNS category eventually make it beyond Phase 3. In the eyes of major pharmaceutical companies, the risk of failure is simply too great
“It costs more than $1 billion to get a drug to market,” Dr. Krystal says, “and often more than half that money is spent in Phase 3.” Given the daunting statistics, “how many of those failures in Phase 3 can a company tolerate?” he asks. “I think the general sense has been that companies feel that the risk/benefit in neuroscience drug development is not acceptable. That’s why some of the biggest pharmaceutical companies have decided over the last decade to pull out; they’re no longer committing resources to developing psychiatric drugs.”
Reversing this trend has been an urgent objective of researchers studying the brain and psychiatric illness. As far back as 2010, BBRF Scientific Council member Steven M. Paul, M.D., and colleagues asked in a paper appearing in the journal Nature Reviews Drug Discovery: “Given that the vast majority of drug candidates are destined to fail...can they fail faster and [therefore] less expensively?” In a nutshell, that is the question the NIMH’s Fast-Fail approach was designed to answer
FAST-FAIL‘S FIRST COMPREHENSIVE TEST
Dr. Krystal and Diego A. Pizzagalli, M.D., a 2017 BBRF Distinguished Investigator and 2008 Independent Investigator at Harvard University/ McLean Hospital, led “Fast- Fail” research in mood disorders that resulted in two published papers in 2020, one appearing in Nature Medicine, the other in Neuropsychopharmacology. They and colleagues subjected a candidate drug made by Johnson & Johnson to early Phase 2 testing, in a protocol that was carefully designed, per NIMH Fast-Fail guidelines, to respond to potential weaknesses in the conventional Phase 2 testing process.
Dr. Krystal explains that conventional Phase 2 studies often recruit too small a patient sample to indicate reliably whether success in meeting the trial’s endpoints will actually result in a therapeutic effect. This raises the odds of subsequent failure when the patient sample is enlarged in Phase 3. Failure risk also rises in Phase 3, he notes, because of another commonplace Phase 2 feature: these studies typically don’t test a specific hypothesis related to why the drug is believed to be promising. For example, a proposed antidepressant drug will be tested in a limited number of depressed patients in Phase 2, with the “endpoint” being defined as a certain amount of reduction in depression symptoms. “Trials of this kind don’t really care about how the drug works so much as whether it has a positive effect.”
This approach has many defenders. After all, the “mechanism of action” of many valuable drugs that have long been on the market remains uncertain. Among them are the SSRI and SNRI antidepressants (such as Prozac, Lexapro, Paxil, Effexor, etc.) that have been taken by tens of millions of Americans. First approved in 1987, these drugs have helped many; yet between one-third and one-half of those who take them do not have a sustained therapeutic response. Because the mechanism through which these drugs affect depression remains unclear, no one can be sure why they don’t help some patients.
Making the matter more difficult, depression is a complex illness, in the dual sense that it is thought to affect many different aspects of brain biology, and that these impacts are believed to be the consequence of a wide array of causative mechanisms, which most likely vary from patient to patient.
The Fast-Fail approach tackles this problem in part by focusing not on broad illnesses like depression, as defined in psychiatry’s Diagnostic & Statistical Manual (DSM) but rather on important symptoms like anhedonia which may affect patients across a number of different illnesses. This “symptom” focus reflects another NIMH initiative, which establishes “Research Domain Criteria” (RDoC). These criteria are intended to provide meaningful biological frameworks for the study of pathologies involved in psychiatric illness. RDoC stresses aspects of behavior—for example, the ability of the brain to learn from past experience, or the ability to register and seek rewards—that pertain to multiple disorders. Learning and reward processing, for instance, are fundamental brain operations and can be disrupted in depression and addiction. Studying these operations may enable researchers to link pathologies across diagnoses in brain systems that are involved in generating symptoms like anhedonia.
TESTING A DRUG FOR ANHEDONIA
Drs. Krystal, Pizzagalli and colleagues designed a Phase 2 trial for an anhedonia drug candidate using the following approach. First, following the NIMH’s Fast-Fail concept, they sought to test an existing compound that already had been through preclinical testing and subsequently had been proven safe in Phase 1 human trials. Further, they sought a drug that had been shown in prior research to “hit” its biological target.
The question to be answered in their Phase 2 trial was: in people with anhedonia, does hitting the target at a known “safe” dosage actually change brain biology? Even more precisely, does it change brain biology in a way that supports the thesis for the drug’s development? If this could not be demonstrated, then by Fast-Fail standards, the drug would “fail”—before another dollar, much less hundreds of millions, were spent on it. Those precious resources could be spent testing another drug with earlystage promise.
The drug selected by Drs. Krystal, Pizzagalli and team, called JNJ-67953964, was already understood from past animal and human experiments to block one of the several naturally occurring receptors for opioids in brain cells, called kappa-opioid receptors (KORs). This was considered interesting because of other preclinical research suggesting that activation of the KOR receptor blocks the release of dopamine in a region of the brain called the ventral striatum. Such release is thought to be correlated with the ability to seek and experience pleasure. The thesis behind the new drug was that blocking the receptor whose activation prevents dopamine from being released might help restore the brain’s “pursuit-of-pleasure” circuitry.
Thus, Drs. Krystal, Pizzagalli and colleagues set out in their Fast-Fail trial to determine whether or not such brain-circuit impacts could be seen in actual patients with anhedonia symptoms.
The patients—86 in all, half of whom received the KOR-blocking drug, and half a placebo—were recruited across six different U.S. testing sites. Although all of the participants had symptoms of anhedonia, and the majority had major depressive disorder, other participants had diagnoses of bipolar disorder, generalized anxiety disorder, social anxiety disorder, panic disorder, and PTSD. The trial was randomized and double-blinded, so that neither patients nor their doctors knew who was receiving drug or placebo.
UNCONVENTIONAL ENDPOINT
Importantly, unlike conventional Phase 2 trials, the primary endpoint for the Fast-Fail trial did not concern the impact of the drug on patients’ anhedonia symptoms (or on depression, with which it is often associated). Rather, it was to gauge the drug’s impact (if any) on rewardrelated neural activation in a part of the ventral striatum called the nucleus accumbens. One of the core hubs of the brain’s reward system, it is located in the middle of the brain and involved in motivation, anticipating and pursuing rewards, and the ability to learn from rewards.
Participants took the KORblocking drug or placebo over an 8-week period. Before the trial began and after it ended, each participant was given a computergenerated task to perform while activity in the ventral striatum was being measured with an fMRI scanning machine. The task measured anticipation of a reward, in this case, monetary gain, based on a computer game.
Compared with those who took placebo, participants who received the KOR-blocking drug showed increased activation in the ventral striatum when expecting a monetary gain.
Another key finding was that participants’ levels of ventral striatum activation at baseline—before they took the drug or played the computer game—predicted the degree to which activation in their ventral striatum would change (if at all) during the trial.
The correlation was strongest for those who received the KORblocking drug—those with higher activation levels before the trial turned out have the greatest increases in activation after 8 weeks of taking the Johnson & Johnson drug. This suggests that the level of activity in the ventral striatum at baseline might serve as a biomarker—pointing to those, in advance of treatment, most likely to benefit from the drug.
As Drs. Krystal and Pizzagalli point out, these results, while gratifying to them and to leaders at the NIMH, may not have satisfied those who prefer to measure clinical trial effectiveness with the classic indicator: how much the drug helped relieve patients’ symptoms. Here, they noted, was a great irony. It turned out that the KOR-blocking drug, in secondary analyses, did have an observable effect; it did lower anhedonia symptoms, as gauged by a standard clinical measure. It also improved an objective measure of anhedonia, as assessed with a computerized task performed outside the MRI scanner, which assessed participants’ ability to change behavior after having received rewards. In the conventional sense, the Johnson & Johnson drug might be said to have “passed” this Phase 2 test.
NEW PERSPECTIVES ON ‘FAILURE‘ AND ‘SUCCESS‘
But as Dr. Krystal explains, this isn’t what he, Dr. Pizzagalli, and colleagues set out to discover. “Does it work?” in the Fast-Fail context means: “Did the drug hit its biological target, and did hitting that target change brain biology in ways consistent with the hypothesis driving the drug’s development? In this case, that hypothesis was confirmed: hitting the target (the KOR receptor in neurons) did change activation in the brain’s reward system (measured by increased ventral striatum activation).
But isn’t it better still that the drug also seemed to reduce anhedonia? Of course, the researchers acknowledge. But what if such benefit had not been observed? The entire approach, in a sense, centers on what action to take if a Phase 2 drug candidate does not yield a clinical benefit in patients. Would that mean that the risk of continuing with development was too high?
Perhaps. But recall the potential problems with Phase 2 trials. Was the 86-patient sample in the Fast-Fail test of the KOR-blocker sufficiently large to reliably determine the effects of the drug on clinical measures of anhedonia? Or what if the dose given, while known to be safe in people, had been too low to generate a change in symptom intensity? Not knowing the answer, would the drug’s developer invest millions more in another multi-year effort to test the drug at higher dosages? Faced with such decisions, many companies have decided not to proceed.
The converse case is also relevant: to see a reduction in symptoms in Phase 2 with a small number of patients is encouraging, but does not guarantee that in a much larger Phase 3 trial, costing hundreds of millions, similar benefits would be observed. Drugs “promoted” from Phase 2 based on small sample sizes have often failed in Phase 3, and that problem runs to the heart of why major pharmaceutical companies stopped developing psychiatric drugs.
‘SUCH AN IMPORTANT APPROACH’
“There are so many examples of really promising Phase 2 results, statistically significant findings, that then crash and burn when a Phase 3 trial is performed,” says Dr. Pizzagalli. In contrast, he says, consider the advantage of the Fast-Fail approach: “You have a very small patient sample, and you try to see whether the intervention you’re testing has an effect on a specific biological target. If it does, then you have to put down chips and say: These are my milestones. If you don’t see that my drug generates the biological impact by a pre-specified amount (that we agree to be significant), then you don’t progress to Phase 3. If you do see the impact, then you move forward. Some people question this, but I think it is such an important approach.”
Also important to note, says Dr. Pizzagalli, the KOR-blocking drug he and colleagues tested did not show any appreciable therapeutic impact upon depression symptoms other than anhedonia. But there are currently no medicines approved for treating symptoms like anhedonia within larger disorders like depression, and depression is the largest “potential market” for such a drug. So despite the fact that many patients have anhedonia symptoms, the result Dr. Pizzagalli and colleagues obtained still might not be viewed as commercially viable. A developer might have to adopt the idea that it “pays”—whether in moral or monetary terms—to develop a drug that addresses important symptoms to justify investing in Phase 3, as in the case of the KOR-blocking compound.
In the words of Dr. Krystal, the counter-intuitive thing about Fast-Fail is that “the goal is to fail drugs more reliably and definitively; it is not necessarily to succeed. Yes, we love to succeed. But with this method, the idea is to identify as early as we can those drugs that shouldn’t move forward, thereby making it possible to devote precious resources to others which have a better chance of making it all the way through the process, all the way to patients.”
It is not yet known if Johnson & Johnson or any other pharmaceutical company has plans to continue developing a KOR-blocking drug. “We do not know, but I will say that the natural next step would be to go ahead and perform Phase 3 trials in anhedonia,” Dr. Krystal says. He adds, “because of the ‘proof of mechanism’ that our trial provided, it need not be this specific compound. It could be any compound that robustly engages the same target; it should have the same effect.”
A final point: Dr. Krystal suggests that understanding how a drug works before testing it in a much larger group of patients enables researchers to better assess any failure or shortfall in Phase 3. Phase 3 failures, which are always possible regardless of prior results, are most troubling when the drug’s biological mechanism is poorly understood. “Having established proof-of-mechanism,” he says, should decrease the likelihood that any positive impacts (on anhedonia, in this case) in Phase 3 “will be due to ‘non-specific effects’—cases where those who take the drug are helped, but for reasons which may have nothing to do with our theory of why it does.”
The Fast-Fail program is now over at the NIMH, its officials say, but the ideas that set it in motion continue to influence the NIMH’s Experimental Therapeutics Program, a cornerstone of its drug development research effort.
Written By Peter Tarr, Ph.D.
Click here to read the Brain & Behavior Magazine's May 2021 issue